Moderna Vaccine Booster Shot Schedule
The two initial Moderna shots contain 100 micrograms of vaccine each. The LA Times reporter on October 14th 2021 US.

Should You Get A Covid Booster Or Third Dose Cleveland Clinic
Earlier this month the FDA was reportedly considering a half dose of the vaccine for the Moderan booster.

Moderna vaccine booster shot schedule. COVID-19 updates Your Health QA. 19 hours agoBoosters comingModerna JJ booster shots could be available as soon as Friday. Age 18 and have underlying medical.
The booster is administered six months after the second shot of the Pfizer vaccine. 12 hours agoMore Americans will soon be able to get a COVID-19 booster shot. Earlier this month the FDA was reportedly considering a half dose of the vaccine for the Moderna booster.
With many Americans who got Pfizer vaccinations already rolling up their sleeves for a booster shot millions of others who received the Moderna or Johnson Johnson vaccine. Modernas current vaccine shot is a 100-microgram dose. Find information about the vaccine intended for vaccination providers.
But the drugmaker says 50 micrograms ought to be enough for a booster for healthy people. Modernas current vaccine shot is a 100-microgram dose compared. 1 day agoThe eligibility requirements for a booster differ depending on vaccine.
Recipients of the booster. Up to 6 cash back People 65 years and older and residents in long-term care settings should receive a booster shot of Pfizer-BioNTechs COVID-19 vaccine at least 6 months after their Pfizer-BioNTech primary series People aged 50 to 64 with certain underlying medical conditions should receive a booster shot of Pfizer-BioNTechs COVID-19 vaccine. Pfizers COVID-19 vaccine is expected to be authorized first and recommended for kids ages 5-11 in early November.
The booster shot meetings are slated for Oct. Health advisors said Thursday that some Americans who received Modernas COVID-19 vaccine at least six months ago should get a half-dose booster. More data on the effectiveness and safety of Moderna and JJJanssen booster shots are expected soon.
14-15 where the panel will review booster data from both JJ and Moderna. Pfizer and Moderna recipients will be able to get an extra shot six months after their second injection if. There are no data available on the interchangeability of the Moderna COVID19 Vaccine with other COVID19 vaccines to complete the vaccination.
Booster shots are available if you received the Pfizer vaccine more than 6 months ago and are. Booster Shots Coronavirus Coronavirus Vaccine Dr. The Moderna COVID19 Vaccine is administered intramuscularly as a series of two doses 05 mL each 1 month apart.
People in the recommended groups who got the Moderna or JJJanssen vaccine may need a booster shot. With those data in hand CDC will keep the public informed with a timely plan for Moderna and JJJanssen booster. Age 65 Residents and staff in long-term care settings.
Smiths Food and Drug has announced it will begin administering the Moderna COVID-19 vaccine for people 70 years old and older starting on Thursday February 11 2021. A key FDA advisory committee unanimously recommended Thursday giving booster shots of Modernas Covid-19 vaccine to people ages 65 and older and other. Appointments for a second dose should be scheduled.
The CDC now recommends a Pfizer COVID-19 vaccine booster shot for eligible individuals. Ad See required Emergency Use Authorization EUA and safety information. You should schedule the second dose of Moderna COVID-19 Vaccine 28 days after the first dose the recommended interval.
Do not use the 4-day grace period when scheduling appointments. Mallika Marshall Moderna BOSTON CBS An FDA panel voted yes on Thursday to recommend a third dose of the Moderna vaccine. Children 12 and over are already eligible for the Pfizer Moderna.
Ad See required Emergency Use Authorization EUA and safety information. The FDAs Vaccines and Related Biological Products Advisory Committee voted 19-0 to recommend the extra dose for all recipients of the JJ Janssen vaccine. 12 hours agoThe Centers for Disease Control and Prevention late Thursday cleared booster shots of Modernas and Johnson Johnsons Covid-19 vaccines giving people the freedom.
Vaccine advisers to the US Food and Drug Administration voted unanimously Friday to recommend a booster dose of Johnson Johnsons vaccine at least two months after people get the first dose reported CNN. Find information about the vaccine intended for vaccination providers. A booster is currently available for people who received the Pfizer vaccine at least six months ago.
Since the initial vaccines were approved pharmaceutical manufacturers have been working on follow-up shots and the CEO of Moderna just revealed when the company plans to have their booster. Do I need to wait between getting flu shot and COVID-19 vaccine.
Booster Shot U S Begins Study Testing Mix And Match Covid Vaccine Doses
Moderna To Begin Trials Of Covid Vaccine Booster Shots For Variant From South Africa
Moderna Covid Vaccine Booster Shot The Latest On Approval Process Cnet

Moderna Covid Vaccine Booster Produces Robust Response Against Delta

Moderna Plans To Have Covid Vaccine Booster Shot Ready By Fall Cbs News
The Biden Administration May Recommend Covid Boosters After 8 Months Npr

Covid Booster Shot Moderna Says Vaccine Generates Promising Immune Response Against Variants
Covid 19 Booster Shots Health Mil

Covid 19 Vaccine Update Lasalle County

Covid Booster Shots Everything You Need To Know The Brink Boston University
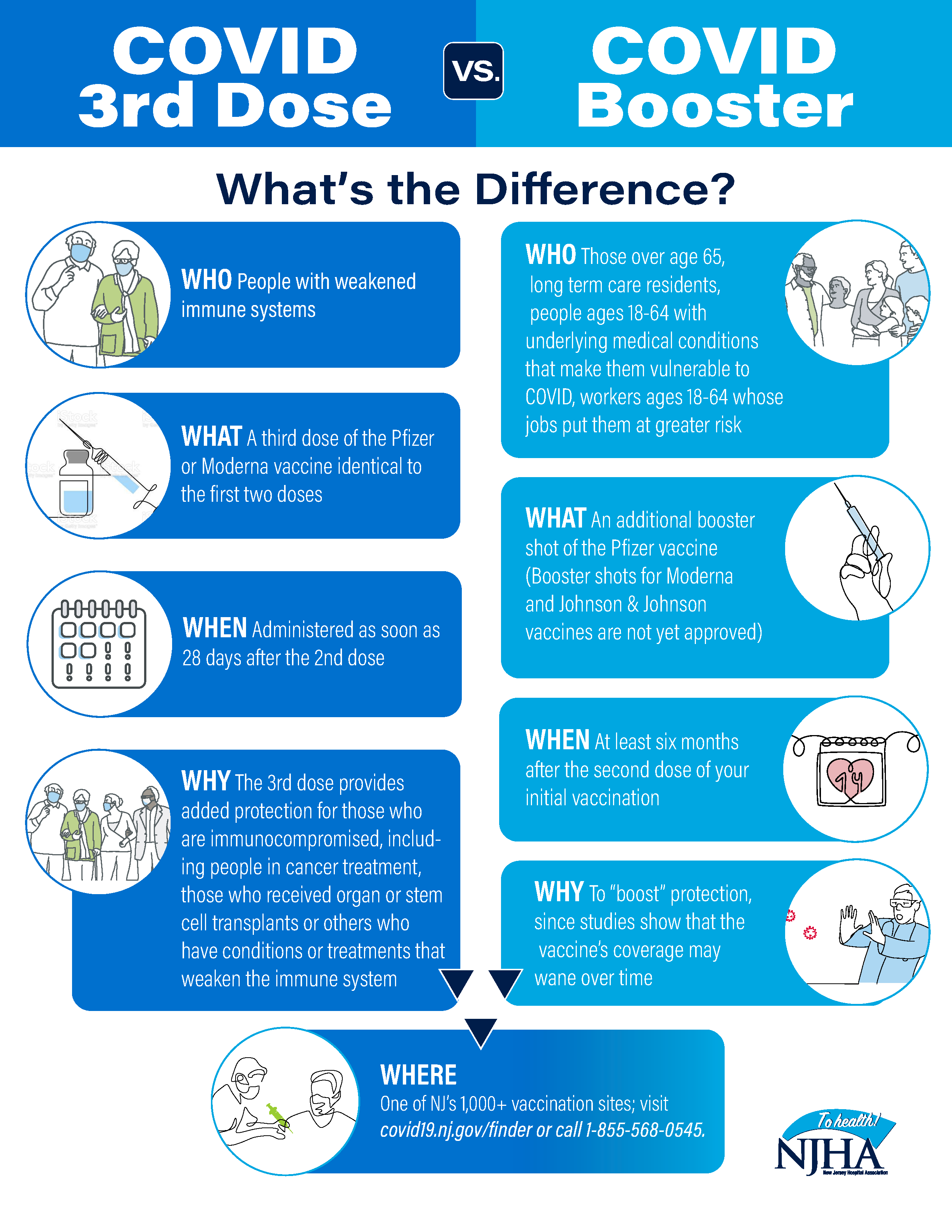
Covid 19 Vaccine 3rd Dose Vs Booster What S The Difference Valley Health System
Fda Clears Moderna And J J Covid Vaccine Boosters Allows Mix And Match Shots
Covid Booster Shot Do I Need It
Covid Vaccine Moderna Hopes To Have Booster Shot Ready By The Fall Says Ceo
Moderna Looks To Test Covid 19 Booster Shots A Year After Initial Vaccination
Lvhn Prepared To Give Pfizer Covid 19 Vaccine Booster Shot To Seniors At Risk Workers Vulnerable Populations Starting Sept 27
Covid Vaccine Ema Backs Pfizer Or Moderna Booster Shots For People With Weak Immunity Euronews

Boosters For Moderna And J J Recipients Not Up For Debate At C D C Panel The New York Times

Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr
